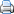Caramel is a beige to dark brown confection made by heating any of a variety of sugars. It is used as a flavoring in puddings and desserts, as a filling in bonbons, and as a topping for ice cream and custard.
The process of caramelization consists of heating sugar slowly to around 170 °C (340 °F). As the sugar heats, the molecules break down and re-form into compounds with a characteristic color and flavor.
A variety of candies, confections, and desserts are made with caramel: caramel apples, pralines, nougats, brittles, crème caramel, and crème brûlée.
Caramelization is the removal of water from a sugar, proceeding to isomerization and polymerization of the sugars into various high-weight compounds. Compounds such as difructose-anhydride may be created from the monosaccharides after water loss. Fragmentation reactions result in low-molecular-weight compounds which may be volatile and may contribute to flavor. Polymerization reactions lead to larger molecular weight compounds, which contribute to the dark brown color.
Caramel candy is a soft, dense, chewy candy made by boiling a mixture of milk or cream, sugar, butter, vanilla essence, and (more common in commercial production) glucose or corn syrup. It can also be made with chocolate. It is not heated above the firm ball stage (120 °C (250 °F)), so there is almost no caramelization. This type of candy is often called milk caramel.
By extension, a candy may be called a "caramel" if it contains such an ingredient. For example, a chocolate bar with a caramel candy filling may be called a "caramel".
Caramel color (150/E150) is a dark, rather bitter-tasting liquid, the highly concentrated product of near total caramelization that is bottled for commercial and industrial use. Beverages such as cola use caramel coloring, and it is also used as a food coloring.
The first scientific account of non-enzymatic reactions involving the browning of sugar was published by the French biochemist Louis Camille Maillard in 1912. He reported that aqueous solutions of amino acids and glucose, or sugar, turned progressively yellow-brown when heated or stored under physiological conditions. This phenomenon, called protein glycation, involves the reaction of a sugar, such as glucose or fructose, with the amino group of proteins to form what is known as a Schiff base. While Maillard’s prediction that this reaction occurs in the human body, particularly in diabetic patients, went unnoticed for the next 50 years, the Maillard reaction became of major interest to researchers in food science and technology. Since the time our ancestors controlled fire, the value of cooking has been recognized for improving the flavor and digestibility of food. It also became apparent that cooked food could be stored longer than raw food. Over time, cooking practices have developed into an art. Food manufacturers have long realized that solutions of amino acids and sugar should not be heated or mixed together. Yet, thermal processes are used in the food industry to improve texture, color, and flavor, and to sterilize and pasteurize, enabling longer shelf-life and improving product safety. While the food industry exploits the Maillard reaction to improve existing products and to develop new products, unfortunately, not all Maillard products endow foods with positive characteristics, such as better flavor and color. For the industry, the goal is to maximize food flavor through the heat-induced Maillard reaction without impairing nutritive value or creating carcinogenic heterocyclic amines, which are formed during high temperature cooking of foods like meat and chicken that contain high amounts of protein. Well-known industrial applications of the Maillard reaction include the production of caramel, cola, coffee, brewed products, infant formulas, and baked products.
| < Prev | Next > |
|---|